Service
The heart of our company and your unmissable partner
Our service team, which can look back at more than 100 years of experience of OTTO JUNKER in industrial furnace building and equipment manufacture, has specialized in supporting customers all over the world with their challenges and projects.
We are there for you 24/7.

Our highly qualified sales team competently deals with customer inquiries and offers the required technical support. It is the first point of contact for customer inquiries of any kind and works closely with the specialized technical departments.
Our Project Managers are professionals in the planning and realization of modernizations and modifications. They work hand in hand with our customers in order to guarantee the smooth running of projects in accordance with the schedule while observing highest quality standards.
In addition to that, our Field Service is specialized in installing our industrial furnaces and equipment on site, commissioning and maintaining it and repairing it when necessary. They are supported by the Service Desk team which not only offers technical support but also plans and coordinates service delegations.
The Spare Parts Team takes care of the procurement and shipment of spare parts and of the repair of our industrial furnaces and equipment and makes sure that our customers always have the required components at their disposal in order to keep their plants in immaculate condition and to guarantee a trouble-free production.
The entire Service Team has built a reputation for excellent customer service and is proud of supporting customers all over the world. We continue our long tradition in industrial furnace building and equipment manufacture and offer our customers innovative solutions to meet their requirements. We at OTTO JUNKER are convinced that our experience and hard work help our customers to reach their production targets using the most reliable state-of-the-art furnace plants in the world.
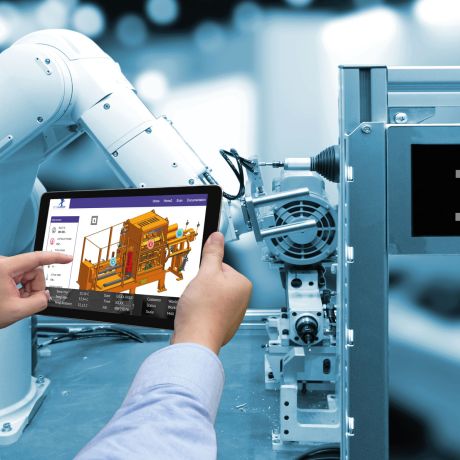
Technical Support
More than anything, to us this means high reachability and worldwide service.
You can always contact us in person through our 24/7 Service Hotline and we can offer you quick support. Further to that, we can access your furnace plant via a remote connection and offer you practical assistance.
The organization of a quick and flexible delegation of our service technicians is of utmost importance to us.
24/7-Service-Hotline

Phone +49 24 73 / 601-555
+49 24 73 / 601-555

E-Mail service@otto-junker.com
service@otto-junker.com